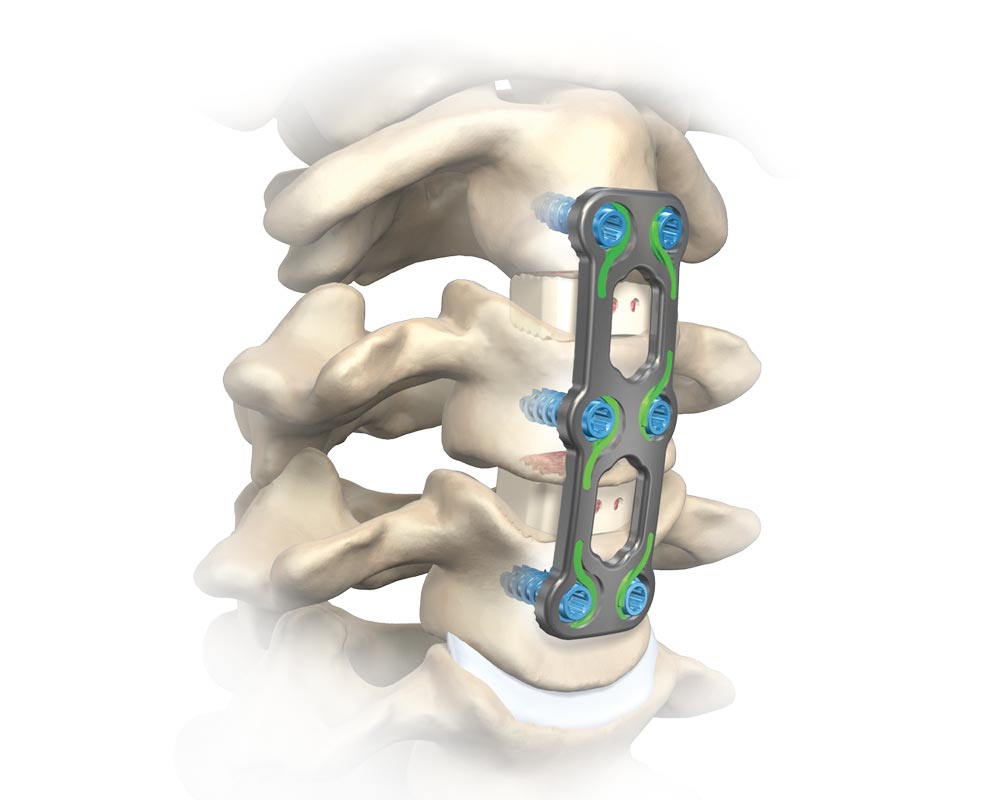
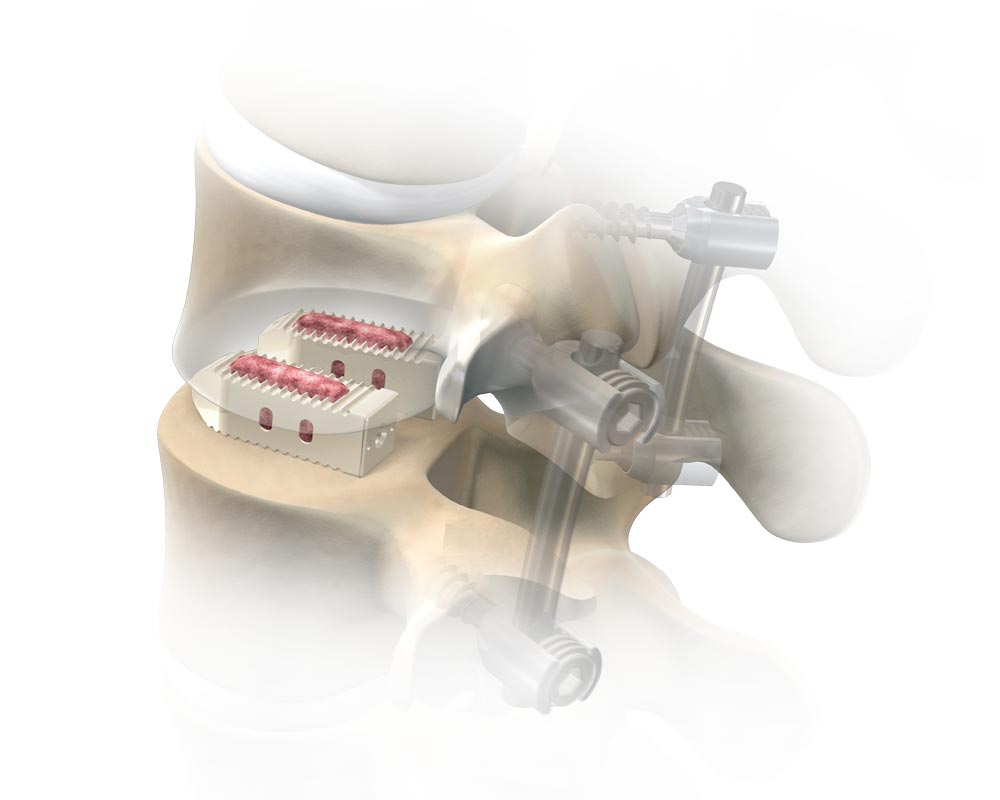
OUR HISTORY
The journey of growth and collaboration in orthopedics and regenerative therapies
Alliance Partners, LLC (dba Alliance Spine) began its journey in 2010 under the leadership of our four executives, with a mission to provide high-quality orthopedic and neurological medical devices. Over the years, we’ve formed strategic partnerships with leading surgeons and state-of-the-art manufacturers, constantly evolving to meet the needs of the regenerative medicine field.
EXCEEDING STANDARTS
Our dedication to quality and regulatory excellence in medical devices
Our Quality Management System ensures that every medical device we design, develop, and deliver is safe, reliable, and meets regulatory requirements. We continuously seek to improve our processes by integrating risk management principles at every stage.
Quality is at the heart of Alliance Spine. We are committed to aligning our products with the highest industry standards, including:
- FDA Registration
- ISO 13485 Certification


ORTHOPEDICS & REGENERATIVE MEDICINE
Delivering advanced medical solutions with proven compliance and safety
We provide innovative, high-quality solutions across orthopedics and regenerative medicine, with strict adherence to regulatory standards. Key areas of focus include:
INNOVATION AND GROWTH
Pioneering solutions for tomorrow's medical challenges
At Alliance Spine, we’re “Inspiring Solutions through Innovation.” Our vision is to continuously innovate and improve our offerings to support the evolving demands of healthcare professionals and patients. We strive to exceed today’s standards and shape the future of orthopedic and regenerative medical solutions through advanced technologies and meaningful partnerships.