BIOLOGICS
Promote AmnioStrip®
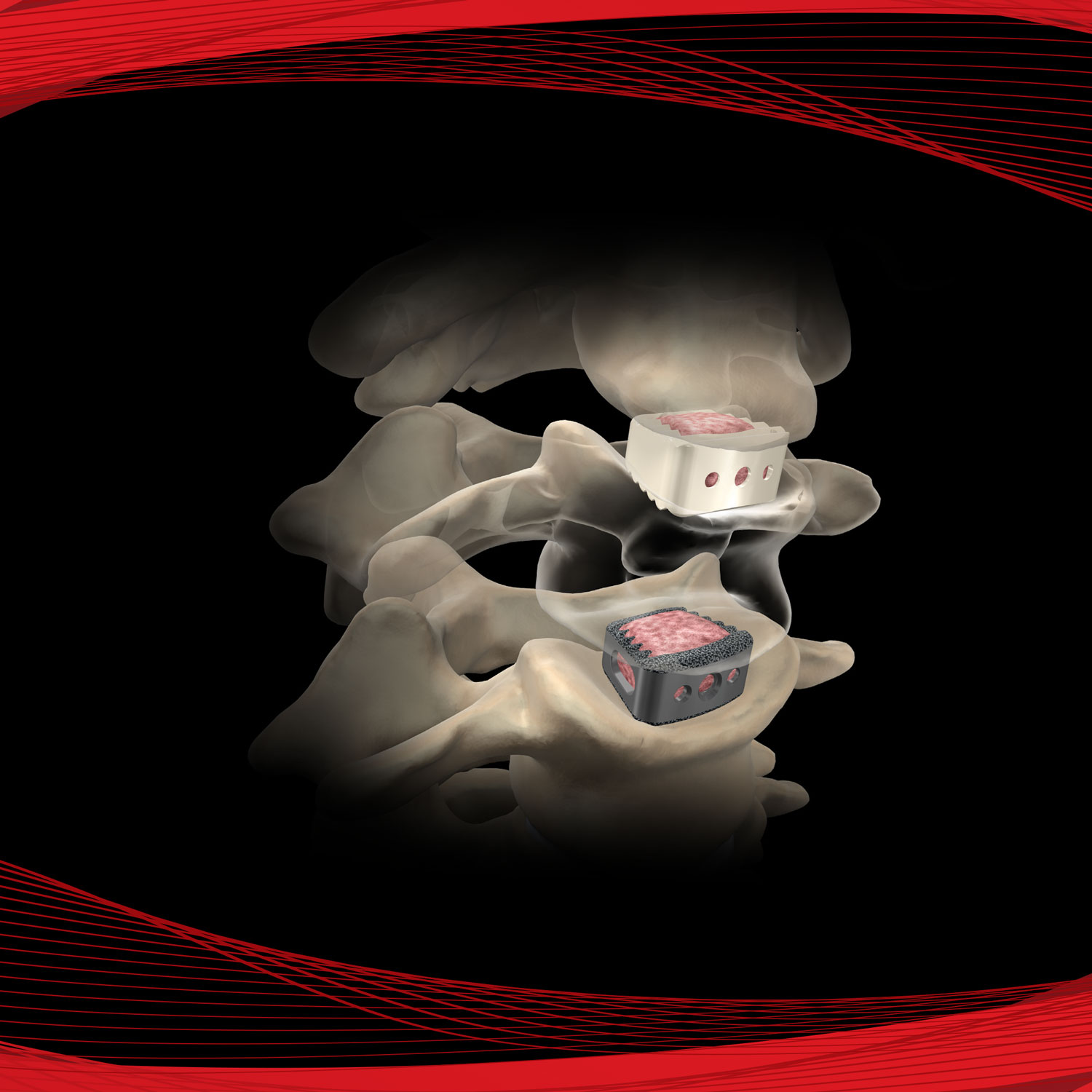
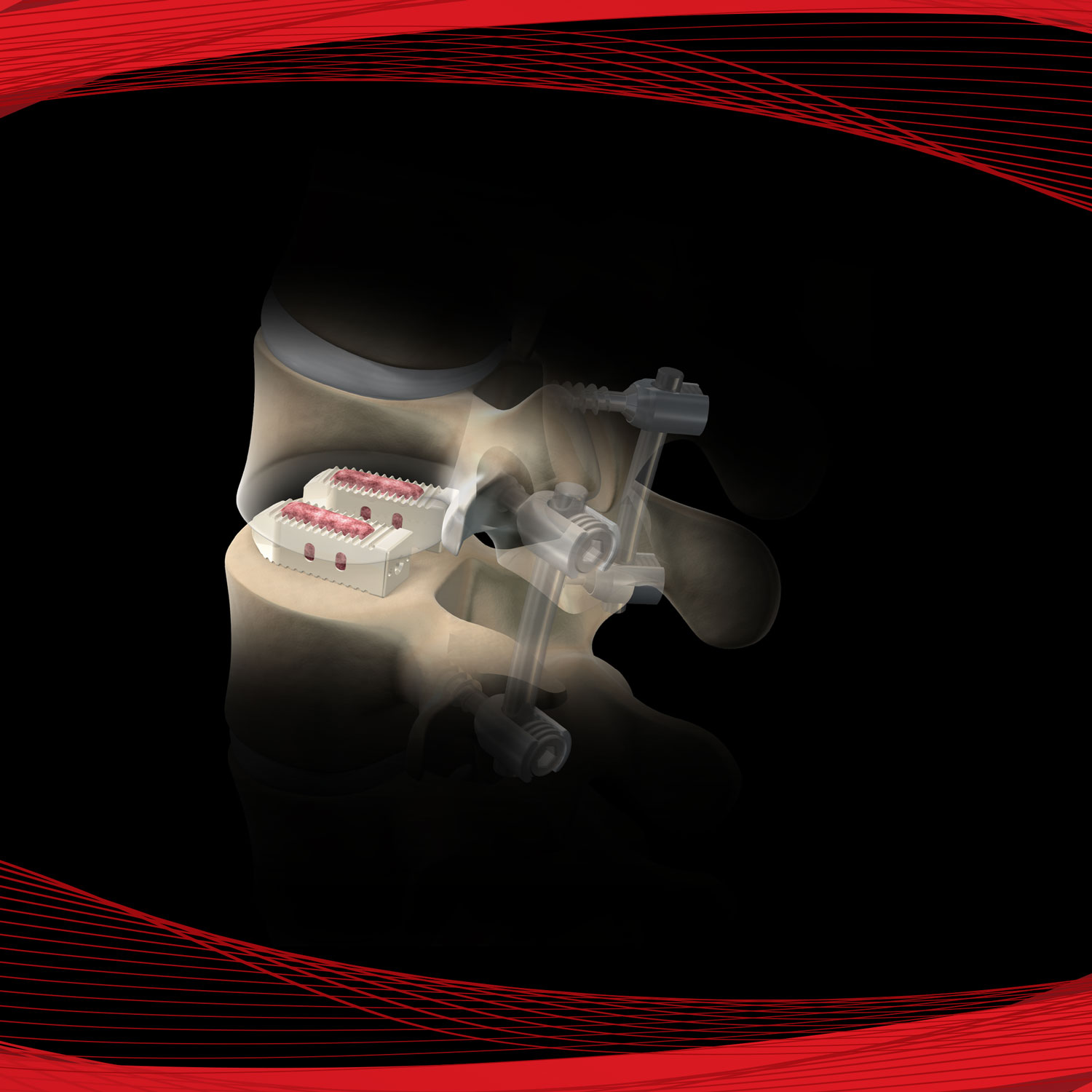
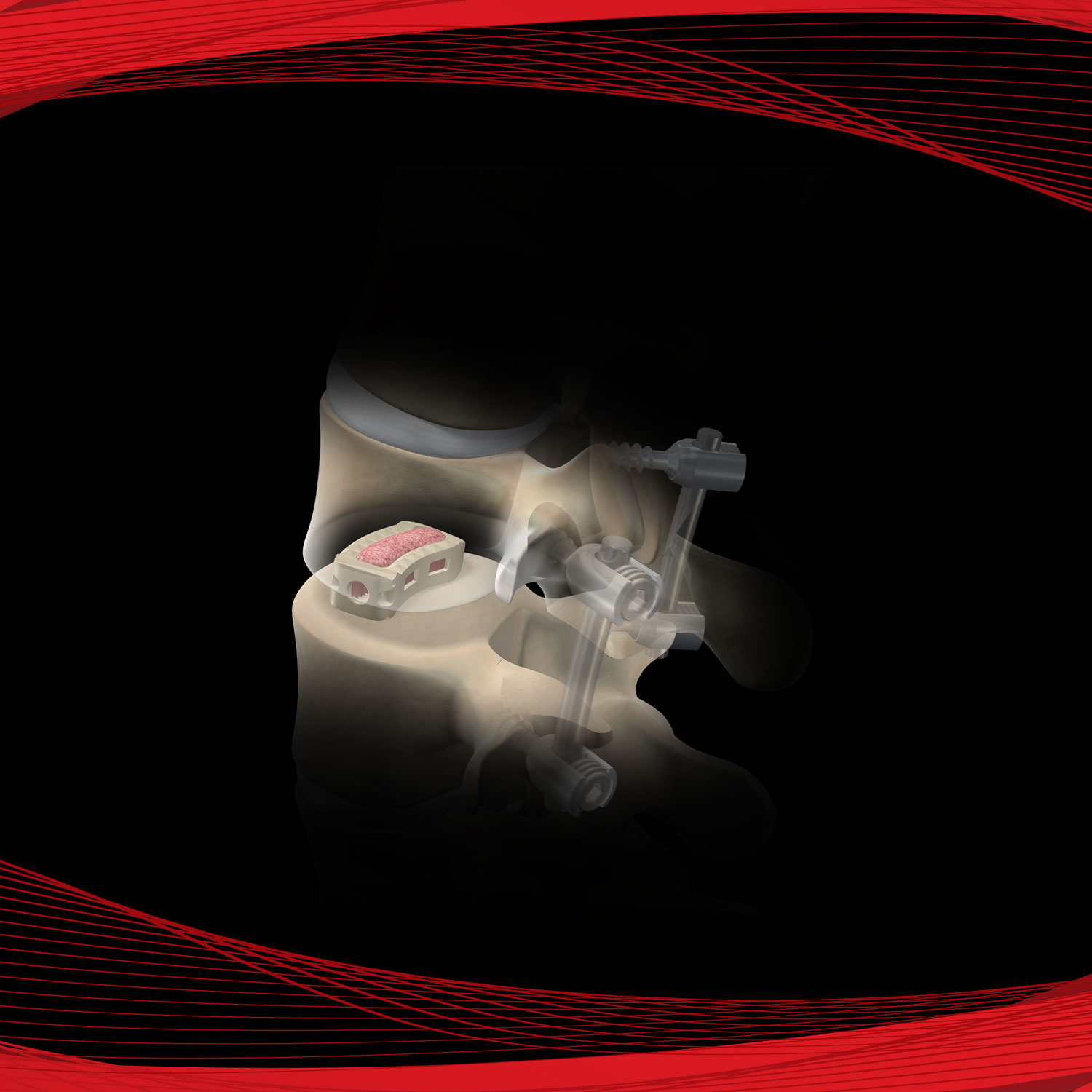
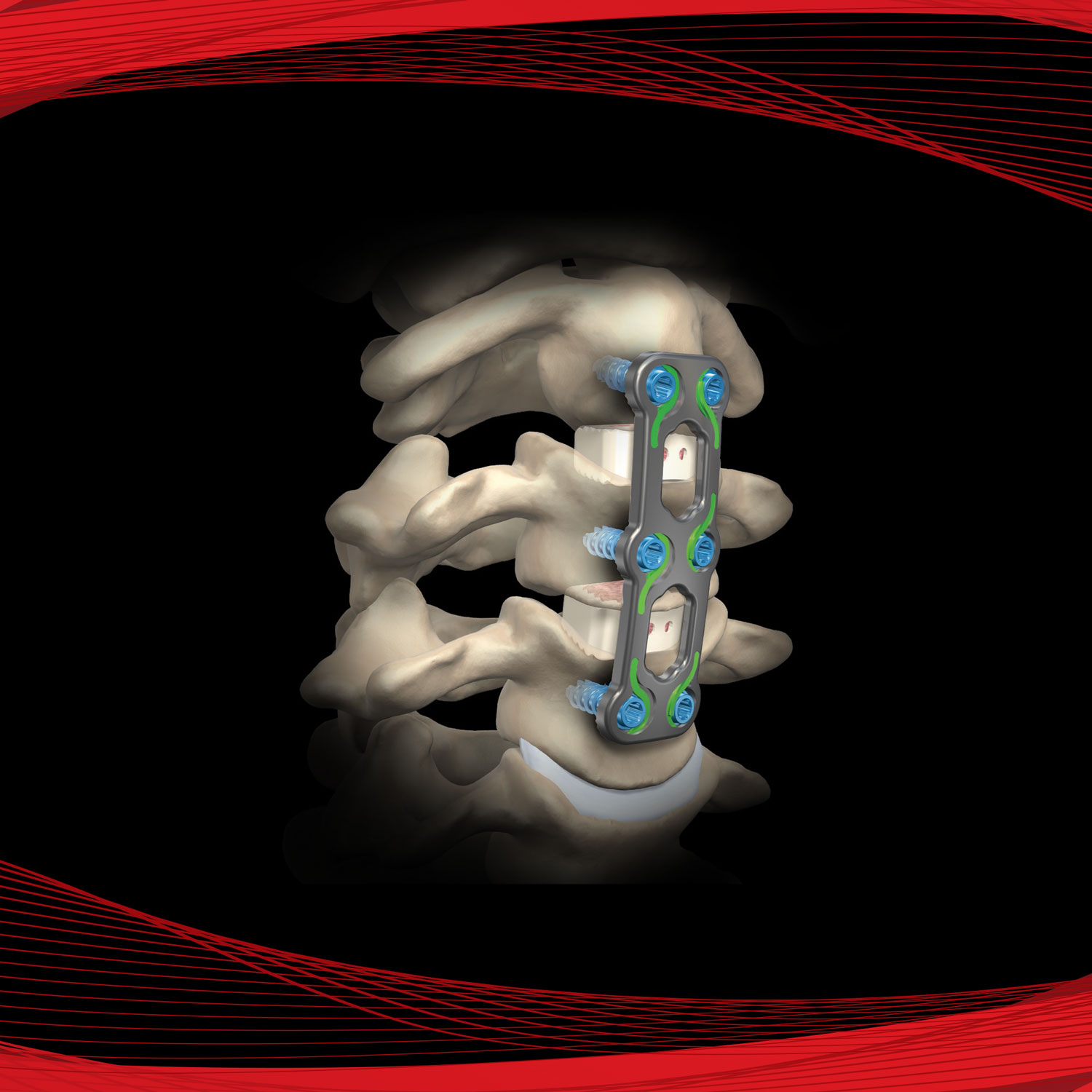
AmnioStrip® is comprised of placental tissue allowing it to be effective at protecting a wide variety of wounds, while simultaneously creating an environment conducive to the regeneration of healthy tissue.
Versatile tissue with application in a variety of surgical procedures. Literature has documented amnion contains collagen, has a variety of growth factors, is compatible and absorbs within the body.
Download brochure
FIND OUT MORE
Interested in learning more about AmnioStrip®?
Discover how we can support your medical applications with proven quality and versatility.
Contact us today to discuss your needs and find the right solution for your practice.
Contact us now