THORACOLUMBAR
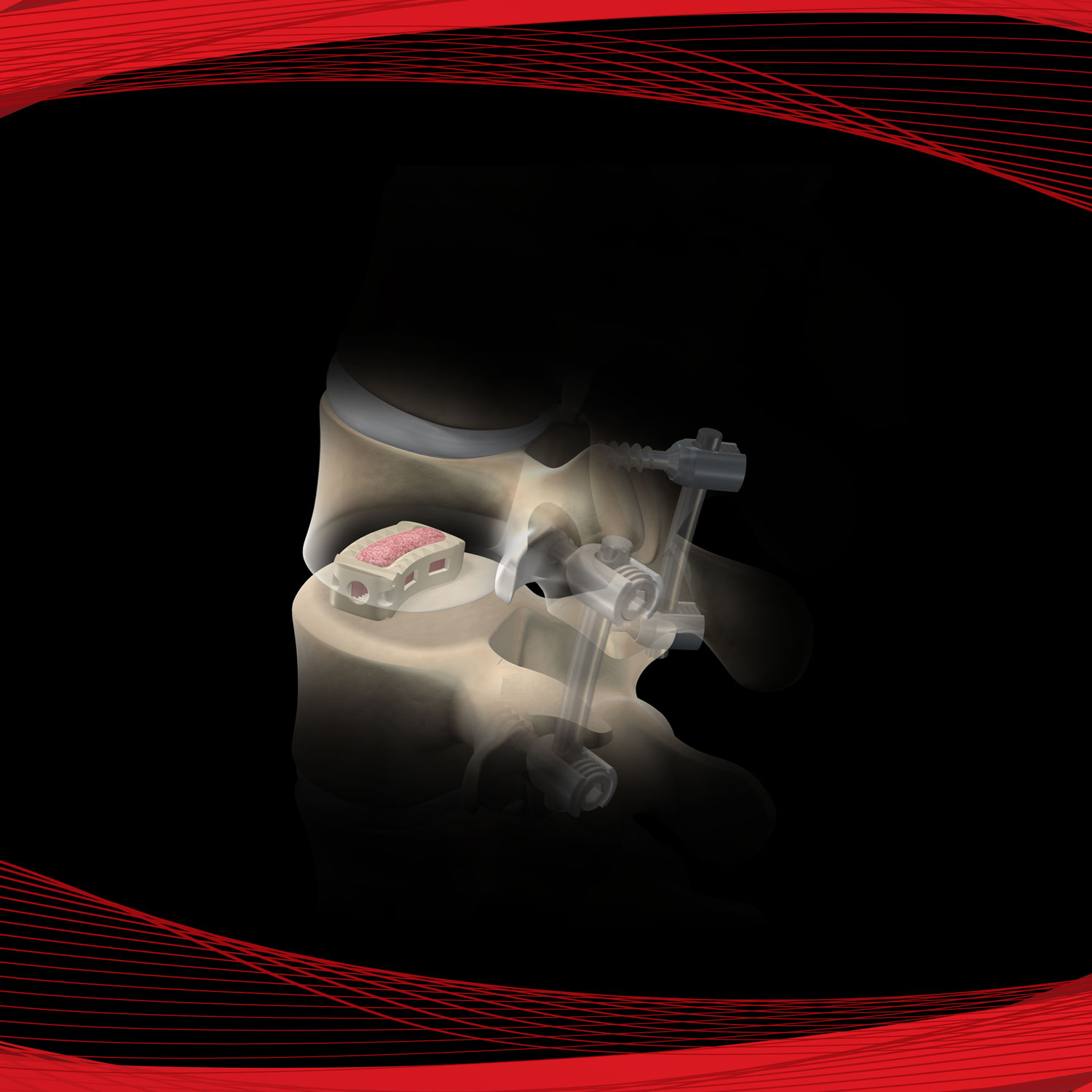
Alamo® T
The Alamo T is used for spinal fusion surgery to provide support and structural stability at the fusion site following discectomy. The device footprint has a hollow center to accommodate bone graft and is implanted via a transforaminal (TLIF) surgical approach. The device is available in various heights and lengths to accommodate variability among patients and the inferior and superior surfaces are designed with ridges to improve fixation and stability and prevent back out and migration.
Download brochure
FIND OUT MORE
Interested in learning more about Alamo® T?
Discover how we can support your medical applications with proven quality and versatility.
Contact us today to discuss your needs and find the right solution for your practice.
Contact us now