BIOLOGICS
Promote OsteoStrip®
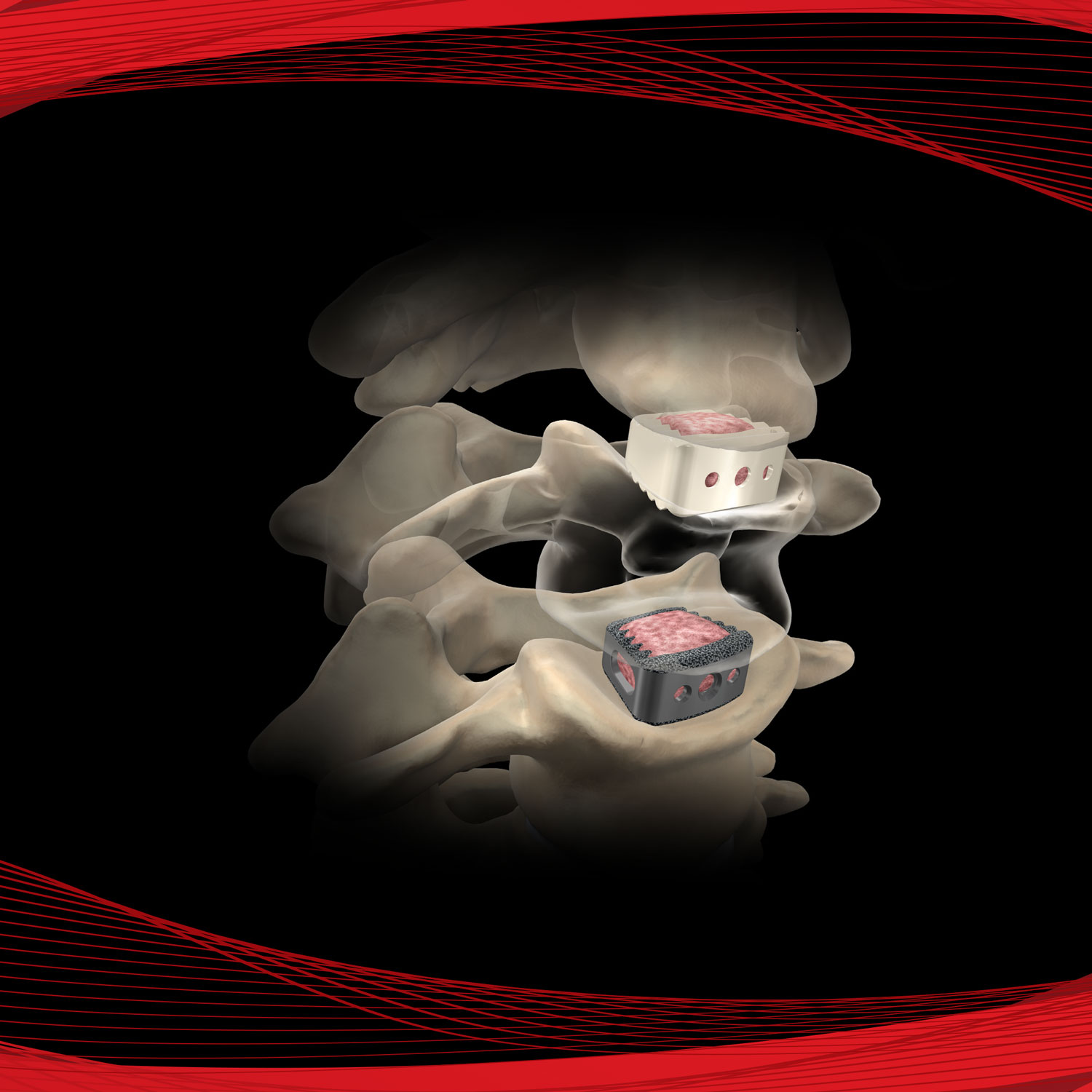
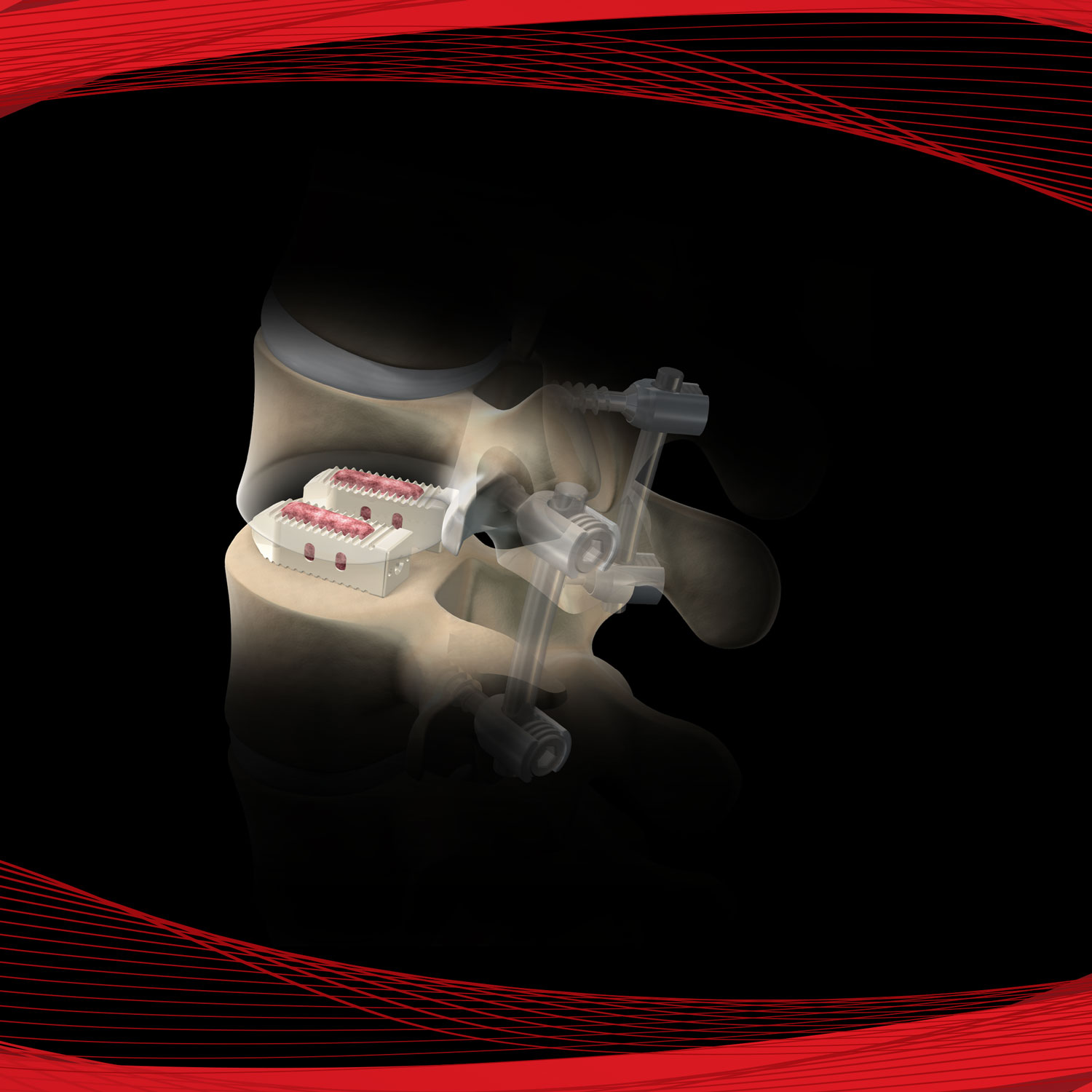
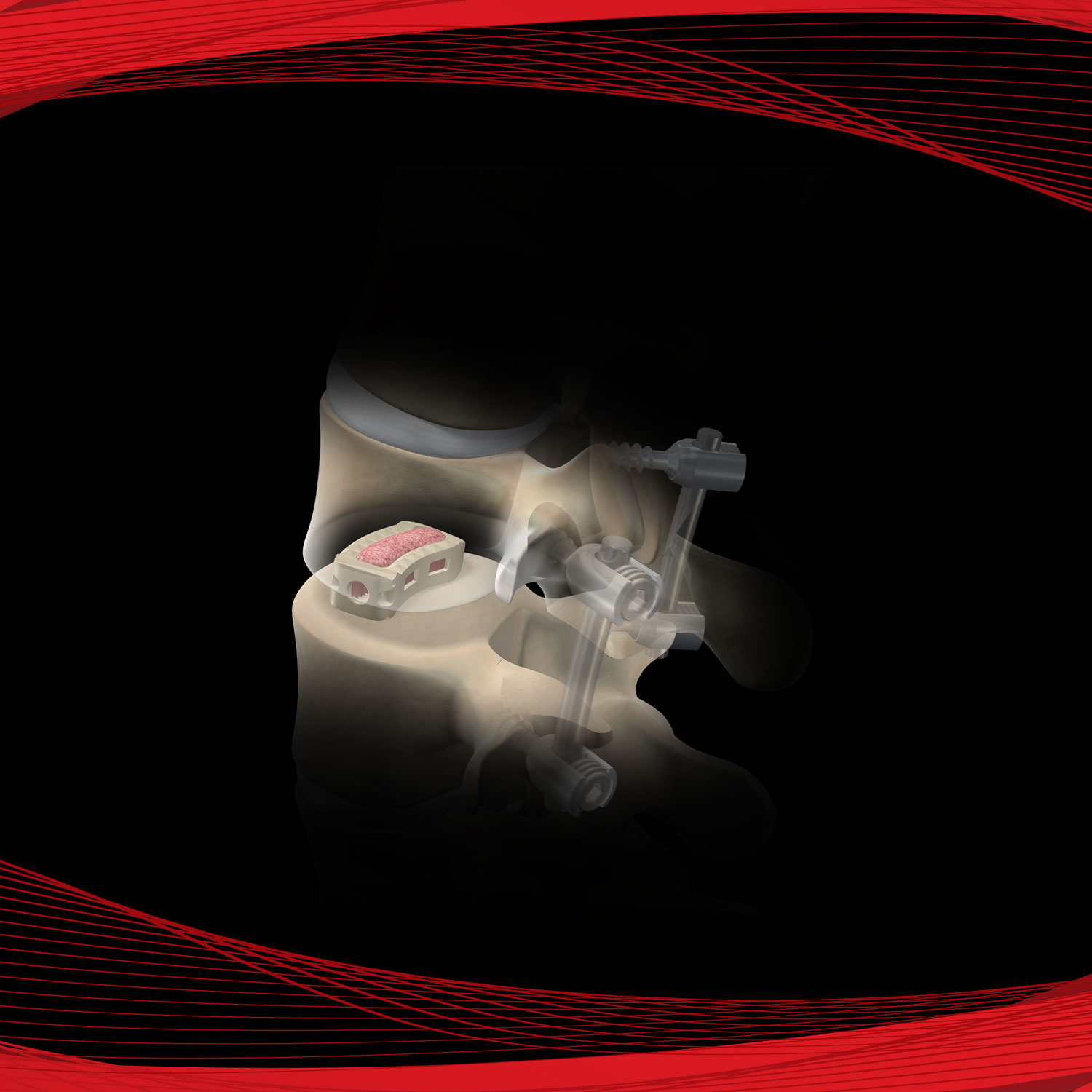
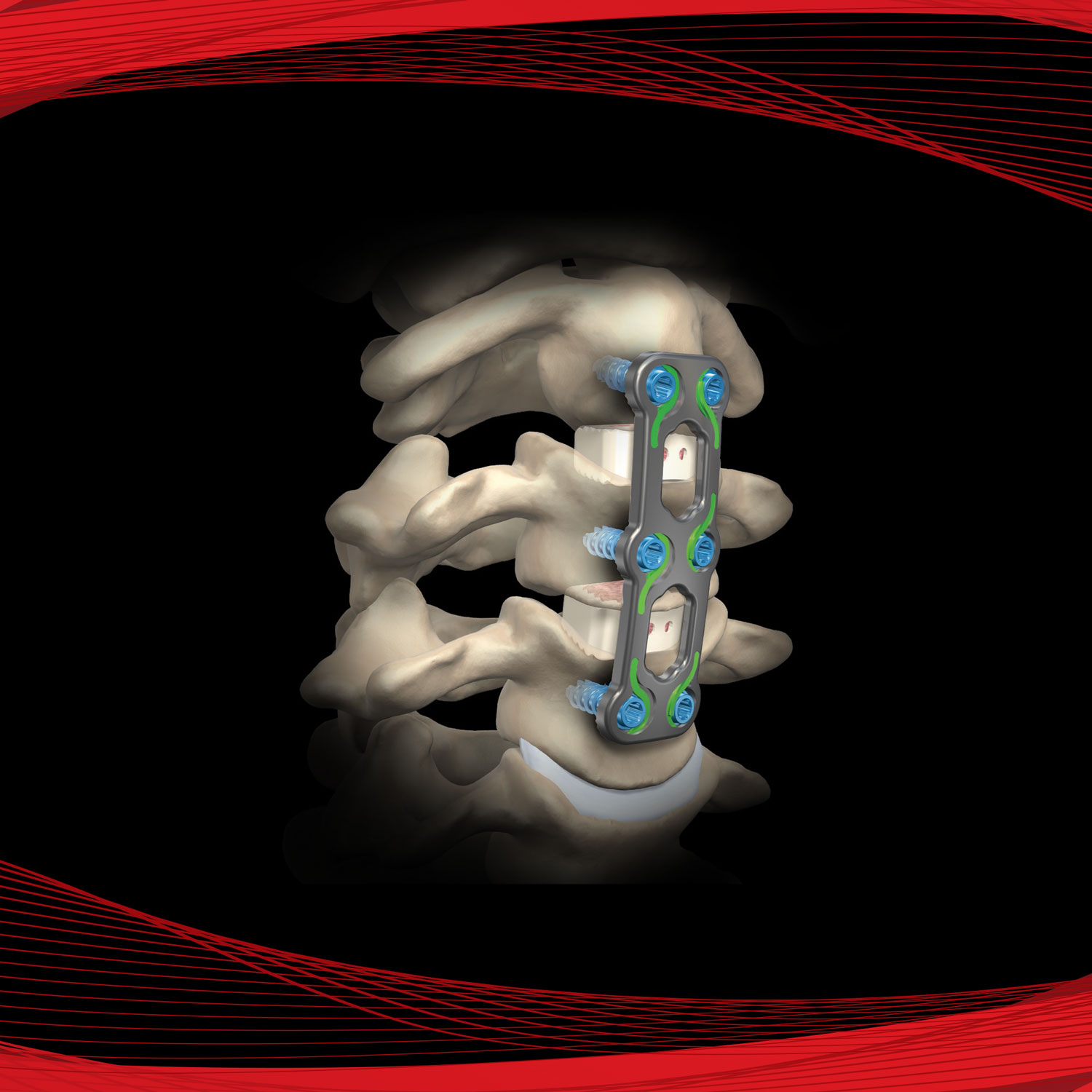
Promote OsteoStrip® is a pliable and absorbable cancellous bone graft that also exhibits osteoconductive and osteoinductive properties. These characteristics are used as a homologous tool for bone grafting in spinal fusions, non-unions, and bone fractures. OsteoStrip® is processed using a next-generation proprietary processing method that maintains the interconnected structure of trabecular bone.
Download brochure
FIND OUT MORE
Interested in learning more about OsteoStrip®?
Discover how we can support your medical applications with proven quality and versatility.
Contact us today to discuss your needs and find the right solution for your practice.
Contact us now